|
| One Hot Summer, by Rosemary Ashton. |
What was it like to live in London through one of the hottest summers on record, with the Thames emitting a sickening smell as a result of the sewage of over two million inhabitants being discharged into the river? How did people cope with the extraordinary heat leading up to the hottest recorded day, Wednesday, 16 June 1858? What did those living or working near the Thames—including at the Houses of Parliament and the law courts in Westminster Hall—do when they found their circumstances intolerable? What did the newspapers say?Ashton proposes to examine London for just a few months in the summer of 1858, providing a snapshot of one moment in Victorian England. Such a microhistory provides insight into the life of mid-19th century Britain.
Microhistory, the study in depth and detail of historical phenomena, can uncover hitherto hidden connections, patterns, and structures. Some events and incidents are revealed over time to have been life changing or nation building. Examples from 1858 are the tackling of London’s sewage and the resultant improvement of public health, Brunel’s engineering feats, the initial laying of the Atlantic telegraph cable, the beginnings of a long process of attaining justice and equality in the matter of marriage and divorce, and the transformation of the miscellaneous medical practice into a proper profession.She focuses on the novelist Charles Dickens, biologist Charles Darwin, and politician Benjamin Disraeli.
A comparatively neglected time in Disraeli’s career can be shown to have been remarkably important in bringing him to prominence. The attention of historians and biographers has focused hitherto on his reckless youth, his racy novels, his controversial journalism, and his late-won success from 1868, when he finally became prime minister. His hard work in the parliamentary session of 1858, particularly in the hectic weeks before the summer break beginning on 2 August, and his success in turning round a hostile press and distrustful colleagues by his efforts, deserve to be acknowledged. In Dickens’s case his painful and self-exposing actions in connection with his failed marriage have been fully discussed, but no detailed account exists of the day-to-day struggles he faced in the long summer which followed his catastrophic error of judgment in advertising his separation from his wife in the early days of June. As for Darwin, though much has been written about his abrupt shock and change of plans on receiving in mid-June Wallace’s letter outlining natural selection, little attention has been paid to the interaction between his family life and scientific work in summer 1858.
This idea of a microhistory sounds fun, and I thought readers of Intermediate Physics for Medicine and Biology might be interested in learning about events in the summer of 1858 that influenced physics, biology, and medicine. So, in this blog post I augment Ashton’s analysis by adding incidents from the world of science.
Charles Darwin (age 48, all ages are as of summer 1858) had been developing his theory of evolution by natural selection for twenty years, since returning to England in 1836 after his famous voyage on the HMS Beagle. Over the years he had told his friends Joseph Hooker (age 41) and Charles Lyell (age 61) about his ideas, but had never published them. Ashton describes how on June 18, 1858 Darwin received a letter from Alfred Russel Wallace (age 35), containing a draft of a paper describing the same idea of natural selection as the mechanism of biological evolution, written while Wallace was collecting biological specimens in the Malay Archipelago. Hooker and Lyell arranged to have some early private writings of Darwin’s, along with the paper by Wallace, published on July 1 at a meeting of the Linnean Society of London.
 |
| On the Origin of Species, by Charles Darwin. |
On June 10, 1858 the botanist Robert Brown died in London, at age 84. In Chapter 4 of IPMB, Russ Hobbie and I write
This movement of microscopic-sized particles, resulting from bombardment by much smaller invisible atoms, was first observed by the English botanist Robert Brown in 1827 and is called Brownian motion.Brown’s death had an interesting impact on the Darwin/Wallace publications. Ashton writes
By a stroke of luck the death of the former president Robert Brown had induced the [Linnean] society to postpone its summer meeting from 17 June, the day before Darwin received Wallace’s letter, to Thursday, 1 July. This meant that Darwin (and Wallace) would not have to wait until September to have their papers made public.One of the most famous scientists in England during 1858 was Michael Faraday (age 65). In Chapter 8 of IPMB, Russ and I discuss electromagnetic induction, which underlies transcranial magnetic stimulation of the brain.
In 1831 Michael Faraday discovered that a changing magnetic field causes an electric current to flow in a circuit.
 | |
| Faraday, Maxwell, and the Electromagnetic Field, by Forbes and Mahon. |
As Faraday’s health and mental faculties declined, he began to relinquish his various responsibilities at the Royal Institution, finally handing over the directorship to John Tyndall in 1865. The consequent loss of income, and of his flat, would have been a worry, but in 1858 Prince Albert, a great admirer, had asked the queen to put a house at Hampton Court at his disposal. Faraday had refused at first, fearing the high cost of repairs, but the queen said she would pay. He and Sarah [his wife] moved in, and the new house became his last home.Although his research career was winding down, Faraday was still a great science communicator. On June 12, 1858 he gave a RI lecture “On the relation of gold to light,” about light scattering from gold colloids (nowadays we would call them gold nanoparticles). He was also famous for his Christmas lectures, which he gave annually throughout the 1850s.
Faraday’s work in electricity and magnetism was carried on by the young James Maxwell (age 27), who was married on June 2, 1858 in Aberdeen, Scotland. That year, Maxwell published his paper “On Faraday’s Lines of Force” (although it had been read before the Cambridge Philosophical Society in late 1855 and early 1856). Forbes and Mahon write
In February 1857, [Maxwell] decided to send a copy of his paper “On Faraday’s Lines of Force” to the great man [Faraday]. No doubt, he did so with some trepidation… He needn’t have worried. As we’ve seen, Faraday’s response was grateful, gracious, and charming. The two had at once formed a rare bond.In the 1860s Maxwell continued his research on electromagnetism, and eventually developed the four Maxwell’s equations that rival Darwin’s theory of evolution as the most significant scientific contribution of the 19th century.
 |
| A Thread Across the Ocean, by John Steele Gordon. |
In a long submarine cable, immersed in a conducting medium—saltwater—the current if often very low, sometimes no more than ten mircoamperes. (The current in a standard incandescent lightbulb is about 100,000 times as great.) The standard galvanometers then available were often inadequate to detect a signal coming through a cable that would be two thousand miles long. So Thomson—half Einstein, half Edison—developed a much better one. He took a very small magnet and attached a tiny mirror to it. Both together weighed no more than a grain. He suspended the magnet from a silk thread and set it in the middle of the coil of very thin insulated copper wire.
When the faint current flowing through the cable was allowed to flow through the copper coil, it created a magnetic field. This caused the magnet, with its attached mirror, to deflect. Thomson simply directed a beam of light from a shaded lamp onto the mirror and allowed it reflection to hit a graduated scale.
In June of 1858 two ships—the Agamemnon and the Niagara—attempted to meet in the middle of the Atlantic Ocean,
splice together the two halves of the cable, and then each pay out the cable as they sailed toward shore: the Niagara toward Newfoundland and the Agamemnon toward Ireland. However, a terrible storm struck the North Atlantic that month, nearly capsizing the Agamemnon with Thomson on board and aborting the mission.
On Sunday, June 20, the storm unleashed a fury such as few sailors ever see and even fewer live to tell about. The caption feared that the coil on the deck, working against its restraints, might break lose and smash through the side, undoubtedly causing the ship to founder.
A second try several weeks later proved more successful. On August 16, the first transatlantic telegraph message was sent between Queen Victoria in England and President James Buchanan in the United States. Unfortunately, the cable soon failed, and it was not until some years later that reliable telegraph service was established across the Atlantic.
Based on his basic research discoveries and his contributions to the telegraph, Thomson became a scientific hero. Gordon writes
In 1892, William Thomson became the first British scientist to be raised to the peerage, when Queen Victoria created him Lord Kelvin of Largs. He has been known ever since as Lord Kelvin. In 1908, the year after he died, the Kelvin temperature scale, devised by him in the 1850s, was named in his honor.
The absolute temperature scale, with Kelvin’s name attached to the unit of temperature, appears throughout IPMB.
Still another notable Victorian physicist was George Stokes (age 36), who at that time was the Lucasian Professor at Cambridge University (a position held earlier by Isaac Newton and later by Stephen Hawking). IPMB often uses Stokes’ law for the viscous force of a small sphere in a fluid. Stokes and Thomson were close friends, and their many letters are preserved. I provide a few excerpts from these letters during late 1857 and 1858.
2 College, Glasgow
Dec. 23, 1857
My Dear Stokes
That principle, in the hydrodynamics of a “perfect liquid”, which I first learned from you, is something that I have always valued as one of the great things of science, simple as it is, and I now see more than ever its importance. One conclusion from it is that instability, or a tendency to run to eddies, or any kind of dissipation of energy, is impossible in a perfect liquid (a fluid with neither viscosity nor compressibility)... [several pages follow with many equations]...Some of the simplest applications of the theory are very interesting: for instance the... case of a circular disc or oblate spheroid, moving... in a perfect [liquid]...
As to Faraday’s magneto-optic experiment, I think my argument that it must depend on a peculiar state of motion induced by magnetic influence (Proceedings R. S. June or July 1856) is unanswerable. Have you considered it?...
It seems like old times for me to be writing you so long a letter, and I am afraid you will be less disposed to be so bored. Your redress simply be not to read it.
With best wishes for a “Merry Christmas” of which there can be no doubt now, I remain
Yours always truly
William Thomson
Stokes responded,
69 Albert Street Regent's Park London N.W.
Feb. 12, 1858
My Dear Thomson,
I have been so very busy of late that your letter has remained for a long time unanswered. I now set to answer it, though I have still got plenty of work before me...
Without having a decided opinion either way I have always inclined to the belief that the motion of a perfect incompressible liquid, primitively at rest, about a solid which continually progressed, was unstable... [pages of math...]
In speculating a good while ago (in fact no great time after Faraday’s discovery) as to the cause of magnetic rotation I naturally tried rotations of the luminiferous ether as suggested by Ampere’s theory...
Yours very truly
G. G. Stokes
Finally, late in 1858, Stokes wrote
Oct 5/58
My Dear Thomson,
... It is a great pity to see the [transatlantic] cable in its present state after apparently so successful a laying down. Still the thing has been done and even if this should be utterly lost the matter will not I presume rest there.
I did not go to Leeds this meeting [The British Science Association met in Leeds in 1858]. On the morning of the 27th my wife was safely delivered of a fine boy. She is going on very well but I am afraid her complete recovery will be slow.
Yours very truly
G. G. Stokes
James Joule (age 39) was yet another English physicist of the Victorian era. His name appears repeatedly in IPMB because the unit of energy is named after him. In the 1840s Joule had done pioneering work on the mechanical equivalent of heat and the conservation of energy, and in the 1850s had collaborated to explain the Joule-Thomson effect. In 1858 he was in a train wreck while traveling home from London. Although unhurt, the accident made him reluctant to travel, somewhat isolating him from the scientific community.
 |
| Gray’s Anatomy. |
A major event in medicine occurred during the summer of 1858: the publication of the first edition of Gray’s Anatomy. In his article “Happy Birthday, Gray’s Anatomy,” Adrian Flatt (Proc. Bayl. Med. Cent., 22:342–345, 2009) writes
Anatomy Descriptive and Applied was first published in London in the summer of 1858 by two young demonstrators of anatomy in St. George’s Hospital at Hyde Park Corner… These two young men were very different. Henry Gray [age 31] wrote the text; he was 4 years older than Henry Vandyke Carter [age 27], who drew all the illustrations…
The print number of 2000 books had been decided, page size was fixed, and all the paper purchased. Considerable adjustments were successfully made and by mid May 1857, the work was going well but was to be interrupted by the absence of Gray. He had received an invitation to “attend” the Duke of Sutherland on his private yacht sailing around England and Scotland and at the estate at Dunrobin Castle for the next 6 months, from June to November 1857. This was manna from heaven for Gray; service for such an aristocrat would be of enormous help to his practice. Carter continued work on the book, of which the final proof corrections were done in late June or early July 1858, in time for the book to be available for students arriving in September.Gray died at age 34, just three years after publication of his textbook, of smallpox. Apparently the relationship between Gray and Carter was strained. Flatt states that
Gray never gave Carter one penny from all the royalties the early editions of the book earned.
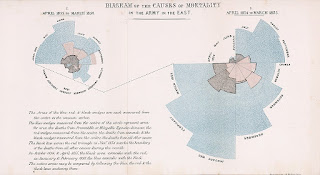 |
| Diagram of the causes of mortality in the army in the East (1858). |
Another leading figure of Victorian health care was Florence Nightingale (age 38), the founder of modern nursing. In 1858 Nightingale published Notes on Matters Affecting the Health, Efficiency, and Hospital Administration of the British Army. Founded Chiefly on the Experience of the Late War. Presented by Request to the Secretary of State for War. This work contained a color statistical illustration called “Diagram of the Causes of Mortality in the Army of the East” that showed that epidemic disease—which caused more British deaths during the Crimean War than battlefield wounds—could be controlled by nutrition, ventilation, and shelter. The infographic became known as Nightingale’s “coxcomb.” Her achievements in statistics were so remarkable that in 1858 she was selected as the first woman fellow of the Royal Statistical Society. Two years later she established her nursing school at Saint Thomas’ Hospital in London.
Another noteworthy happening in medicine was the death of John Snow (age 45) on June 16, 1858 (London’s hottest day of that steamy summer). Snow was best known for figuring out the source of the Broad Street cholera outbreak in 1854, when he demonstrated that cholera was being spread through contaminated water from one specific pump. He also studied using ether as an anesthesia during surgery.
 |
| The Ghost Map, by Steven Johnson. |
In June 1858, a relentless early-summer heat wave produced a stench of epic proportions along the banks of the polluted Thames. The press quickly dubbed it the “Great Stink”... [Yet] the rates of death from epidemic disease proved to be entirely normal. Somehow the most notorious cloud of miasmatic air in the history of London had failed to produce even the slightest uptick in disease mortality... It's easy to imagine John Snow taking great delight in [this] puzzling data... But he never got the opportunity. He had suffered a stroke in his office on June 10... and died six days later, just as the Great Stink was reaching its peak above the foul waters of the Thames.
Joseph Lister (age 31) was in Edinburgh in 1858, studying the coagulation of blood and inflammation. In the 1860s he developed antiseptic surgery, and later relocated to London. In their article “Joseph Lister: Father of Modern Surgery” (Can. J. Surg., 55:E8–E9, 2012), Dennis Pitt and Jean-Michel Aubin claim that
it was Lister’s application of germ theory to the care of surgical patients that laid the foundation for what surgeons do now. He directed the minds of physicians and surgeons to the vital necessity of keeping wounds clean and free of contamination.
Finally, in 1858 Elizabeth Garrett Anderson (age 22) was a young woman dreaming of making a career in medicine. She eventually became the first female doctor in the United Kingdom.
Ashton believes that microhistory provides valuable insight into Victorian England. Near the end of her Prologue she concludes
Intense scrutiny of the lives of these men [Dickens, Darwin, and Disraeli, plus Brown, Faraday, Maxwell, Thomson, Stokes, Joule, Gray, Nightingale, Snow, and others] over a short period of a few months allows us to make fresh threads of connection between each of them and the larger society in which they lived, all at a time of public events which provided to be of lasting national importance.