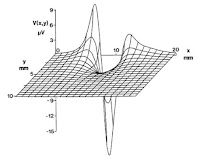
9. Figure 7.13. I helped create this figure when I was in graduate school. Russ asked my PhD advisor John Wikswo if he could supply two figures showing the extracellular potential (Fig. 7.13) and magnetic field (Fig. 8.14) produced by an axon. Wikswo asked me to do the calculations, and he had an illustrator in the lab produce the final drawing.
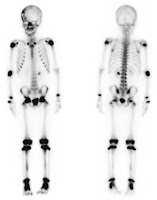
8. Figure 17.19. This scintillation camera bone scan of a 7-year-old boy is spooky, with ghostly radioactive hot spots. It is one of the many medical images Russ obtained from colleagues at the University of Minnesota. In this case, Bruce Hallelquist provided the photo. IPMB is much the richer for all the images provided by Russ’s friends.
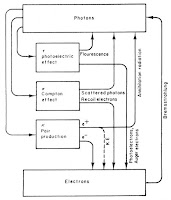
7. Figure 15.15. This figures illustrates the transfer of energy between photons and electrons. I like how it summarizes much of the chapter about the Interaction of Photons and Charged Particles with Matter in a single drawing.
6. Figure 14.24. New in the 4th edition of IPMB, this figure illustrates the blackbody radiation spectrum. It clarifies why the spectrum appears different when plotted versus frequency compared to when plotted versus wavelength.
5. Figure 12.12. This illustration defining the projection is critical to understanding tomography. Russ and I liked it so much that we considered using it on the cover of the 4th edition of IPMB, until Springer decided to go with their own cover design that didn’t include a figure from the book.
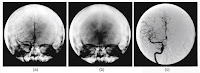
4. Figure 16.23. This image, obtained using digital subtraction angiography, is another medical illustration provided by one of Russ’s colleagues at the University of Minnesota (Richard Geise). I chose it because it is stunningly beautiful.
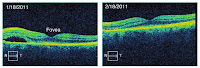
3. Figure 14.16. Color! This optical coherence tomogram of the retina was supplied by Kirk Morgan. A few figures in IPMB go beyond black and white, but this is the only one in glorious full color.
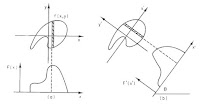
2. Figure 12.6. I like this magnetic resonance image of the brain because it helps build insight into how an image and its Fourier transform are related. It is the first of a series of six images in Chapter 12 prepared by Tuong Huu Le (University of Minnesota, also thanks to Xiaoping Hu) that, by themselves, provide a short course in image processing.
And the winner is….
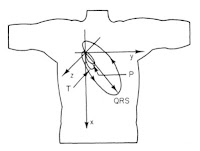
1. Figure 7.16. This picture of the direction of the dipole during the cardiac cycle nicely summarizes the electrocardiogram. My career has focused on the bioelectric behavior of the heart, so it is fitting that my top pick builds on that theme. The reason I chose it, however, is because it was on the cover of the first edition of IPMB, which I used in my first medical physics course taught by John Wikswo at Vanderbilt University.