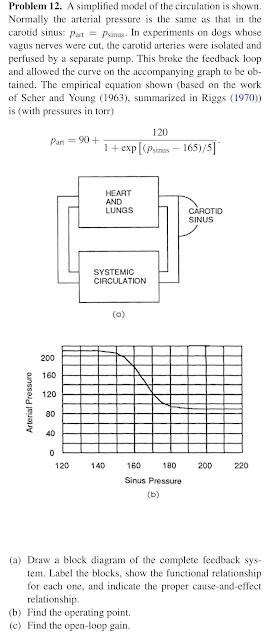
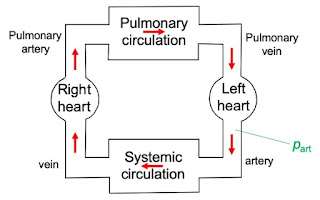
In Chapter 7 of
Intermediate Physics for Medicine and Biology,
Russ Hobbie and I discuss the
electrocardiogram. Today, I present twelve
ECGs that everyone should know. I’ve drawn them in a stylized and schematic way, ignoring differences between individuals, changes from beat to beat, and noise.
When I taught
medical physics at
Oakland University, my lecture on ECGs was preceded by a lesson on
cardiac anatomy. If some
anatomical terms in this post are unfamiliar, I suggest reviewing the
Texas Heart Institute website.
1. Normal Heartbeat
 |
The electrocardiogram (ECG).
|
Electrocardiograms are plotted on graph paper that consists of large squares, each divided into a five-by-five grid of small squares. The horizontal axis is time and the vertical axis is voltage. Each large square (or box) corresponds to a fifth of a second and a half of a millivolt.
The normal ECG contains three deflections: a small P wave associated with depolarization of the atria, a large narrow QRS complex associated with depolarization of the ventricles, and a T wave associated with repolarization of the ventricles.
A normal heart rate ranges from 60 to 100 beats per minute. The ECG below repeats every four boxes, so the time between beats is 0.8 seconds or 0.0133 minutes, which is equivalent to a heart rate of 75 beats per minute.
 |
Normal heartbeat.
|
2. Sinus Bradycardia
A sinus bradycardia is a slow heart beat (slower than 60 beats per minute, or five boxes). The term sinus means the sinoatrial node is causing the slow rate. This node in the right atrium is the heart’s natural pacemaker. Other than its slow rate, the ECG looks normal. A sinus bradycardia may need to be treated by drugs or an implantable pacemaker, or it may represent the healthy heartbeat of a trained athlete who pumps a large amount of blood with each beat.
 |
Sinus bradycardia.
|
3. Sinus Tachycardia
A sinus tachycardia is the opposite of a sinus bradycardia. It’s a fast heart beat (faster than 100 beats per minute, or three boxes). The rapid rate arises because the sinoatrial node paces the heart too quickly. A sinus tachycardia may trigger other more severe arrhythmias we discuss later.
 |
Sinus tachycardia.
|
4. Atrial Flutter
During atrial flutter a reentrant circuit exists in the atria. That is, a wave front propagates in a loop, constantly chasing its tail. The nearly sinusoidal, small-magnitude signal in the ECG originates in the atria. Every few rotations around the circuit, the wave front passes through the atrioventricular node—the only connection between the atria and the ventricles—giving rise to a normal-looking QRS complex (the T wave is buried in the atrial signal).
 |
Atrial flutter.
|
5. Atrial Fibrillation
Atrial fibrillation is similar to atrial flutter, except the wave fronts in the atrium are not as well organized, propagating in complicated, chaotic patterns resembling turbulence. The part of the ECG arising from the atria looks like noise. Occasionally the wave front passes through the atrioventricular node and produces a normal QRS complex. During atrial fibrillation the ventricles do not fill with blood effectively because the atria and ventricles are not properly synchronized.
 |
Atrial fibrillation.
|
6. First-Degree Atrioventricular Block
In first-degree atrioventricular block, the time between the end of the P wave and the start of the QRS complex is longer than it should be. Otherwise, the ECG appears normal. This rarely results in a problem for the patient, but does imply that the atrioventricular node is not healthy and trouble with it may develop in the future.
 |
First-degree atrioventricular block.
|
7. Second-Degree Atrioventricular Block
In second-degree atrioventricular block, the P waves appear like clockwork. The signal often passes through the atrioventricular node to the ventricles, but occasionally it does not. Different types of second-degree block exist. Sometimes the delay between the P wave and the QRS complex gets progressively longer with each beat until propagation through the atrioventricular node fails. Other times, the node will periodically drop a beat; for example if every second beat fails you have 2:1 AV block. Still other times, the node fails sporadically.
 |
Second-degree atrioventricular block.
|
8. Third-Degree Atrioventricular Block
If your atrioventricular node stops working entirely, you have third-degree atrioventricular block (also known as complete heart block). A ventricular beat exists because some other part of the conduction system (say, the Bundle of His or the Purkinje fibers) serves as the pacemaker. In this case, the P wave and QRS complex are unsynchronized, like two metronomes set to different rates. In complete heart block, the ventricles typically fire at a slow rate. Sometimes a patient will intermittently go in and out of third-degree block, causing fainting spells.
 |
Third-degree atrioventricular block.
|
9. Premature Ventricular Contraction
Sometimes the heart will beat with a normal ECG, and than sporadically have an extra ventricular beat: a premature ventricular contraction. Usually these beats originate within the ventricle, so they are not distributed through the cardiac conduction system and therefore produce a wide, bizzare QRS complex. As long as these premature contractions are rare they are not too dangerous, but they risk triggering more severe ventricular arrhythmias.
 |
Premature ventricular contraction.
|
10. Ventricular Tachycardia
A ventricular tachycardia is a rapid heartbeat arising from a reentrant circuit in the ventricles. The ECG looks like a series of premature ventricular contractions following one after another. The VT signal typically has a large amplitude, and the atrial signal is often too small to be seen. This is a serious arrhythmia, because at such a fast rate the heart doesn’t pump blood effectively. It’s not lethal itself, but can become deadly if it decays into ventricular fibrillation.
 |
Ventricular tachycardia.
|
11. Ventricular Fibrillation
In ventricular fibrillation, different parts of the ventricles contract out of sync, resulting in the heart quivering rather than beating. A heart in VF does not pump blood, and the patient will die in ten to fifteen minutes unless defibrillated by a strong electric shock. Ventricular fibrillation is the most common cause of sudden cardiac death.
 |
Ventricular fibrillation.
|
12. Asystole
In asystole, the heart has no electrical activity, so the ECG is a flat line. Asystole is the end stage of ventricular fibrillation, when the chaotic electrical activity dies away and nothing remains.
 |
| Asystole. |
Once you master these twelve ECGs, you’ll be on your way to understanding the electrical behavior of the heart. If you want to learn more, I suggest trying the SkillStat six-second ECG game, which includes 27 ECGs. It’s fun.