Myocardial cells are typically about 10 μm in diameter and 100 μm long. They have the added complication that they are connected to one another by gap junctions… This allows currents to flow directly from one cell to another without flowing in the extracellular medium. The bidomain (two-domain) model is often used to model this situation… It considers a region, small compared to the size of the heart, that contains many cells and their surrounding extracellular fluid. It simplifies the problem by assuming that each small volume element contains two domains, intracellular and extracellular.
The bidomain model has become the state-of-the-art representation of the electrical properties of cardiac tissue, and much of my research was focused on it. Les Tung’s 1979 PhD dissertation was one of the first publications to use the model (“A Bi-Domain Model for Describing Ischemic Myocardial DC Potentials,” Massachusetts Institute of Technology). I read his dissertation in graduate school and it had a huge impact on my research. Tung writes
The bidomain structure developed here is a detailed, quantitative realization of the concept of interpenetrating domains, described qualitatively by Schmitt (1969).At about the same time, David Geselowitz and his student Tom Miller developed a similar model. In a 1983 paper (“A Bidomain Model for Anisotropic Cardiac Tissue,” Annals of Biomedical Engineering, Volume 11, Pages 191–206), they also cite the same source.
Schmitt (20) introduced the concept of “interpenetrating domains” based on a consideration of the electrical properties of a region containing many cells. He proposed that each point in the muscle be represented by an intracellular resistivity “representing cytoplasmic impedance of a neighborhood of like cells on a volume normalized basis,” and by a similar extracellular resistivity. The two would be connected at each point by a distributed nonlinear admittivity simulating active cell membrane.
In 1984, Robert Plonsey and Roger Barr published an early paper about the bidomain model (“Current Flow Patterns in Two-Dimensional Anisotropic Bisyncytia with Normal and Extreme Conductivities,” Biophysical Journal, Volume 45, Pages 557–571). They wrote
Because the viewpoint is global rather than cellular (discrete) it is convenient to consider both intracellular space and interstitial space to be continuous and described by the same coordinates (both spaces are necessarily congruent, or, as described by Schmitt (2), “interpenetrating domains”).All three publications cite the same book chapter by Otto Schmitt titled “Biological Information Processing Using the Concept of Interpenetrating Domains” (in Information Processing in The Nervous System, Leibovic, K. N., editor, Springer, Berlin, Pages 325–331). I decided that if this chapter is the true source of the bidomain concept, then I should read it (or reread it, as I remember looking at it decades ago). The interesting feature about the chapter is not what’s in it, but what isn’t. Schmitt never uses these words: bidomain, cardiac, heart, myocardium, syncytium, gap junction, or cable. Instead, the chapter focuses entirely on the nervous system, and never even hints at cardiovascular applications. So, what did Schmitt write that was so influential?
Let us introduce the notion of a local regional electrical vector impedivity representing cytoplasmic impedance of a neighborhood of like cells on a volume normalized basis and similarly represent regional interstitial fluid as an external impedivity with similar normalization and vectorial properties. Connect these two at every point by a distributed, scalar, nonlinear admittivity, simulating typical active cell membrane.Schmitt then presents a more visual description of his idea.
If there is difficulty in comprehending this triple interpenetration of two impedivity and one admittivity domains, think of the following homely illustration. Imagine a three dimensional cubic fly screen of resistance wire as the first impedivity. Notice that another identical screen of perhaps different conductivity could be fitted completely within the first fly screen without touching it. A moderately conductive fluid poured into the fly screen system would, for all practical purposes, connect the two screens everywhere but only in a very limited neighborhood around each paired mesh cell would this conductivity be important.
Schmitt called this the interpenetrating domain model, but nowadays we call it the bidomain model (or sometimes, the bisyncytial model). Ironically, this idea is rarely used to model the nervous system, which was what Schmitt had in mind. It’s most applicable to syncytial tissues: when the cytoplasm of an individual cell is coupled to neighboring cells through gap junctions. Without such intercellular channels, the intracellular domain is not coupled like a “fly screen” but rather consists of uncoupled individual cells. Cardiac muscle is the classic example of a tissue in which all the cells are coupled via gap junctions, so it acts like a syncytium.
Interestingly, the fly screen analogy looks similar to this bidomain resistor illustration, versions of which I've used in many publications.
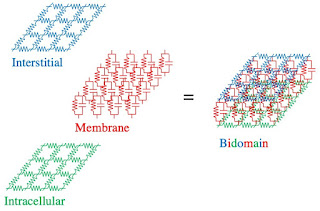 |
| The Bidomain Model (two interpenetrating domains). |
I think it’s a stretch to say that Schmitt is the father of the bidomain model. Perhaps grandfather would better characterize his contribution. He didn’t derive a mathematical formulation of his idea. But he certainly conceived an intuitive picture of two coupled interpenetrating domains, and that influenced later work by Tung, Geselowitz, and Plonsey.
I’ll end with the abstract from a short biography of Otto Schmitt by Jon Harkness (Physics in Perspective, Volume 4, Pages 456-490, 2002), written four years after Schmitt’s death.
A Lifetime of Connections: Otto Herbert Schmitt, 1913–1998
Jon M. Harkness
Otto H. Schmitt was born in St. Louis, Missouri, in 1913. As a youth, he displayed an affinity for electrical engineering but also pursued a wide range of other interests. He applied his multi-disciplinary talents as an undergraduate and graduate student at Washington University, where he worked in three departments: physics, zoology, and mathematics. For his doctoral research, Schmitt designed and built an electronic device to mimic the propagation of action potentials along nerve fibers. His most famous invention, now called the Schmitt trigger, arose from this early research. Schmitt spent most of his career at the University of Minnesota, where he did pioneering work in biophysics and bioengineering. He also worked at national and international levels to place biophysics and bioengineering on sound institutional footings. His years at Minnesota were interrupted by World War II. During that conflict—and the initial months of the Cold War to follow—Schmitt carried out defense-related research at the Airborne Instruments Laboratory in New York. Toward the end of his career at Minnesota, Schmitt coined the term biomimetics. He died in 1998.
Otto Schmitt discussing his work during World War II.











