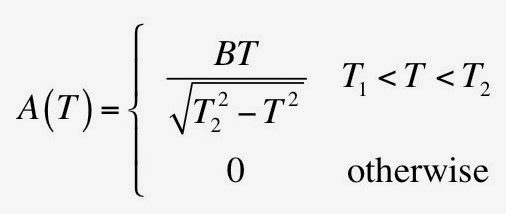|
| The First Steps in Seeing, by Robert Rodieck. |
This book is about the eyes—how they capture an image and convert it to neural messages that ultimately result in visual experience. An appreciation of how the eyes work is rooted in diverse areas of science—optics, photochemistry, biochemistry, cellular biology, neurobiology, molecular biology, psychophysics, psychology, and evolutionary biology. This gives the study of vision a rich mixture of breadth and depth.Rodieck explains things in a quantitative, almost “physicsy” way. For instance, he imagines a person staring at the star Polaris, and estimates the number of photons (5500) arriving at the eye each tenth of a second (approximately the time required for visual perception), then determines their distribution on the retina, finds how many are at each wavelength, and how many per cone cell.
The findings related to vision from any one of these fields are not difficult to understand in themselves, but in order to be clear and precise, each discipline has developed its own set of words and conceptual relations—in effect is own language—and for those wanting a broad introduction to vision, these separate languages can present more of an impediment to understanding than an aid. Yet what lies beneath the words usually has a beautiful simplicity.
My aim in this book is to describe how we see in a manner understandable to all. I’ve attempted to restrict the number of technical terms, to associate the terms that are used with a picture or icon that visually express what they mean, and to develop conceptual relations according to arrangements of these icons, or by other graphical means. Experimental findings have been recast in the natural world whenever possible, and broad themes attempt to bring together different lines of thought that are usually treated separately.
The main chapters provide a thin thread that can be read without reference to other books. They are followed by some additional topics that explore certain areas in greater depth, and by notes that link the chapters and topics to the broader literature.
My intent is to provide you with a framework for understanding what is known about the first steps in seeing by building upon what you already know.
Color vision is analyzed, as are the mechanisms of how rhodopsin responds to a photon, how the photoreceptor produces a polarization of the neurons, how the retina responds with such a large dynamic range (“the range of vision extends from a catch rate of about one photon per photoreceptor per hour to a million per second”), and how eye movements hold an image steady on the retina. There’s even a discussion of photometry, with a table similar to the one I presented last week in this blog. I learned that the unit of retinal illuminance is the troland (td), defined as the luminance (candelas per square meter) times the pupil area (square millimeters).
Like IPMB, Rodieck ends his book with several appendices, including a first one on angles. His appendix on blackbody radiation includes in a figure showing the Planck function versus frequency plotted on log-log paper (I’ve always seen it plotted on linear axes, but the log-log plot helps clairfy the behavior at very large and small frequencies). The photon emission from the surface of a blackbody as a function of temperature is 1.52 × 1015 T3 photons per second per square meter (Rodieck does everything in terms of the number of photons). The factor of temperature cubed is not a typo; Stefan's law contains a T3 rather than T4 when written in terms of photon number. A lovely appendix analyzes the Poisson distribution, and another compares frequency and wavelength distributions.
The best feature of The First Steps in Seeing are the illustrations. This is a beautiful book. I suspect Rodieck read Edward Tufte’s the Visual Display of Quantitative Information, because his figures and plots elegantly make his points with little superfluous clutter. I highly recommend this book.