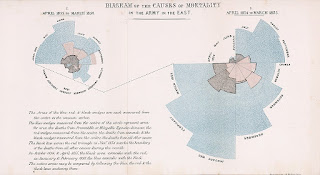
A few weeks ago, I published a blog post about the television show Meeting of Minds. That show from the late 1970s was created and hosted by Steve Allen and featured historical figures as guests in a talk show format. In my earlier post, I wrote that if I were going to have an episode of Meeting of Minds based on Intermediate Physics for Medicine and Biology, it would include guests Alan Hodgkin, Willem Einthoven, Paul Lauterbur, and Marie Curie.
As your Christmas present, I offer you a script for the IPMB episode of Meeting of Minds.
Enjoy!
***********************************************************
 |
| Meeting of Minds, IPMB episode. |
Allen: Good evening. I’m Steve Allen and I’d like to welcome you to this week’s episode of Meeting of Minds. Our guests will all be drawn from the field of physics applied to medicine and biology. We have the French physicist Marie Curie, the English physiologist Alan Hodgkin, the Dutch medical doctor Willem Einthoven, and the American chemist Paul Lauterbur. I’d first like to introduce Alan Hodgkin. [applause]
Hodgkin: [Hodgkin enters from stage right, and sits at the table.] Thank you, thank you. It’s such a pleasure to be here, Mr. Allen.
Allen: The pleasure’s all mine, Dr. Hodgkin. Tell me, I understand you were born in the market town of Banbury, England in 1914, the son of Quakers. How did a Quaker upbringing influence your early life?
Hodgkin: It had a huge influence. As you know, Quakers are pacifists, and I was born just as World War I began. When I was only two, my father, George Hodgkin, traveled to Armenia to try to help the many refuges trying to escape genocide committed by the Ottoman Empire. He tried to return to to Armenia two years later, but ended up dying of dysentery in Baghdad. This was just a few weeks after my brother Keith was born. I was only four when dad died.
Allen: But your Quaker roots didn’t stop you from participating in World War II?
Hodgkin: No, not at all. In fact, I was eager to do my part against the Nazis. I had visited Germany in 1932 and that experience destroyed any pacifist beliefs I might’ve held. During World War II, I worked on radar. In fact, I was on one of the first test flights of a Bristol Blenheim light bomber when it was fitted with our airborne centimetric radar system.
Allen: Going back to your childhood, were there any influences that led you to a scientific career?
Hodgkin: Oh yes. My mother encouraged my scientific interests and so did my Aunt Katie, who used to take me bird watching. In high school, I even got a job surveying rookeries and heronries. I spent many hours wading around in the salt marshes watching birds. This experience kindled my love for science.
Allen: I see you started your career in the biological sciences. Where did you gain the knowledge of the physical sciences that allowed you to work on radar?
Hodgkin: When at college at Cambridge, one of my zoology professors gave me some good advice: study as much math and physics as you can! I also picked up a lot doing student research. And of course, during the war I learned on the job. I’m very interested in learning how my esteemed colleague Willem Einthoven made a similar transition from biology to physics.
Allen: In that case, let’s welcome the father of clinical electrocardiography, Willem Einthoven. [applause]
Einthoven: [Einthoven enters from stage left and sits at the table across from Hodgkin] So good to meet you Mr. Allen. And it is truly a delight to meet the famous Alan Hodgkin, of Hodgkin and Huxley fame.
Dr. Hodgkin, I see we have something in common.
Hodgkin: Oh, what’s that?
Einthoven: We both lost our fathers early in our life. My father, Jacob Einthoven, was a doctor, and died when I was only six. I was not born in the Netherlands, but in Java, which at that time was part of the Dutch East Indies. After dad died, we returned to the Netherlands and settled in Utrecht.
Allen: I understand you studied medicine.
Einthoven: Yes, Mr. Allen. When I was 25 I received my medical degree from the University of Utrecht. Then I became a professor at the University of Leiden, where I spent my career. At that time, I married my first cousin Frédérique Jeanne Louise de Vogel.
Hodgkin: First cousin! [giggles from the audience]
Einthoven: Yes, a wonderful woman. [frowning]
Allen: Like Dr. Hodgkin, your biological and medical research required knowledge of physics and math. How did you learn these subjects?
Einthoven: Through self study, Mr. Allen.
Hodgkin: The best type of learning.
Einthoven: I obtained a textbook by the Dutch physicist Hendrik Lorentz and taught myself differential and integral calculus. Thirty years later, I gave a copy of that book to the American cardiologist Frank Wilson (of the Wilson central terminal) with the inscription “May I send you the excellent book of Lorentz’ Differential- und Integralrechnung? I have learned my mathematics from it after my nomination as a professor in this University and I hope you will have as much pleasure and profit by it as I have had myself.” I also benefited from talks with my brother-in-law Julius, a physics professor at Utrecht. My training and degree was in medicine, but in my heart of hearts I was a physicist.
Hodgkin: Fascinating.
Einthoven: What is truly fascinating is how all the guests tonight contributed to the study of bioelectricity and biomagnetism. I developed the electrocardiogram, and you Dr. Hodgkin figured out how nerves work. I am anxious to meet Dr. Lauterbur, who invented magnetic resonance imaging.
Allen: Then without further ado, let me invite Dr. Paul Lauterbur to join our stimulating discussion. [applause]
Lauterbur: [Lauterbur enters from stage right, and sits next to Hodgkin.] Steve Allen [shakes hand]. Drs. Hodgkin and Einthoven [nods]. So happy to be here. Willem, my ancestors came from over in your neck of the woods. They’re from Luxembourg.
Einthoven: Interesting. Luxembourg is more closely related to France and Germany than the Netherlands, but….
Hodgkin: Ha!
Lauterbur: Yeah, we Americans are a little weak on our geography. I was born and raised in the small town of Sidney, Ohio, just north of Dayton.
Einthoven: And how did you become interested in science, Dr. Lauterbur?
Lauterbur: As a teenager I built my own chemistry laboratory in the basement of our house.
Hodgkin: Nice.
Lauterbur: My high school chemistry teacher realized that I liked experimenting, so he let me do my own chemistry experiments in the back of the room during class.
Einthoven: Such a wise teacher.
Lauterbur: I got my bachelor’s degree in industrial chemistry form Case Institute of Technology in Cleveland, which is now part of Case Western Reserve University. Like Dr. Hodgkin, I served in the army. In the early 1950s I was assigned to the army chemical center in Edgewood, Maryland. They let me spend part of my time using an early nuclear magnetic resonance machine. It didn’t do imaging…
Allen: Of course not! You invented imaging.
Lauterbur: …but NMR machines are important for chemical identification. I actually published four papers by the time I was discharged.
Hodgkin: And where did you do your famous work on imaging?
Lauterbur: I was at Stony Brook University for 22 years.
Einthoven: On Long Island?
Lauterbur: Yes, Willem, your geography’s better than mine [quiet laughter from the audience].
Allen: I look forward to hearing about your development of MRI later in our discussion, but now I would like to introduce our last guest, Marie Curie [enthusiastic applause, louder than for any of the other scientists].
Curie: [Curie enters from stage left and sits next to Einthoven] Thank you. Thank you so very much.
Einthoven: The honor is ours, Dr. Curie. Why, you are the only one of us who has an element named for them.
Curie: Yes, element 96 is named curium.
Lauterbur: While on the topic of geography, I seem to associate you with two countries, Marie: France and Poland. Which is your home?
Curie: Well, I was born and raised in Warsaw, which at that time was part of the Russian Empire. It was only when I was 24 that I went to Paris, and I spent the rest of my life in France. But I never lost my Polish heritage. I made sure my daughters learned the Polish language, and we went on trips to Poland. And Dr. Einthoven, you will be interested to know that I managed to get an element, polonium, named after my beloved homeland [scattered applause from the audience].
Einthoven: Nicely done.
Hodgkin: And how did you get started in science, Dr. Curie?
Curie: “Dr. Curie.” That still seems strange to hear. You see, it wasn’t as easy for a young lady to start a career in science as it was for you men.
Allen: I’m sure it wasn’t.
Curie: Also, my education was difficult because my parents were involved in uprisings to gain Polish independence. We lost much of the family fortune. My father was a physics teacher. When Russia eliminated laboratory instruction from Polish schools, dad brought all the lab equipment home for us kids to use. The Russians finally fired my father. Like Drs. Hodgkin and Einthoven, I lost a parent when I was young. For me, it was my mother, who died of tuberculosis when I was ten.
I couldn’t pursue higher education then, because I was a woman…
Einthoven: So unfair.
Curie: I did become involved with the clandestine Flying University, a Polish patriotic institution that admitted women. Eventually my sister Bronislawa and I made a deal. I stayed in Poland and made money to pay for her medical studies in Paris. In exchange, she agreed to help me pay for my education two years later.
I got a job tutoring for some wealthy relations of my father. I fell in love with one of the sons, Kazimierz, but his family wouldn’t allow a marriage to a poor tutor. Kazimierz later became a famous mathematician. When he was an old man and I had died, he would come sit and stare at my statue at the Radium Institute.
I eventually was able to go to Paris and join my sister. Still, life was hard. I studied so hard I often forgot to eat! I hit the books all day and tutored in the evenings to pay the bills.
Hodgkin: How did you meet your husband Pierre Curie?
Curie: We met through our mutual love of science. When Pierre proposed, I was reluctant to accept because I planned to return to Poland. Pierre said he would give up his distinguished physics career to join me! [applause from the audience]. We eventually were married. One smart aleck called me “Pierre’s greatest discovery” but his work in magnetism was very important in its own right.
Allen: Now I would like to switch the conversation to the science that made each of you famous. Dr. Hodgkin, you first. Tell us a little about your work with the squid nerve axon.
Hodgkin: I’d love to. Yes, Dr. Curie, some people think they’re so witty. In my case, one wise guy said that “it’s the squid who really deserved the Nobel Prize” [polite laughter from the audience]. I must admit he had a point. We conducted our experiments using the giant axon of the squid, which is up to one millimeter in diameter.
Einthoven: One millimeter!? For a nerve axon, that is huge!
Hodgkin: Yes, that’s the point. It was big enough that we could stick electrodes down its length.
Curie: Who is “we,” Dr. Hodgkin?
Hodgkin: I worked mainly with my collaborator, Andrew Huxley. He was a little younger than me and started as my student, but eventually became a true collaborator. He was an excellent experimentalist, but one of his greatest strengths was his skill in mathematics.
Lauterbur: Do go on, Alan. What did ya do with those axons?
Hodgkin: We developed a technique called the “voltage clamp” in which we used two electrodes, one to measure the voltage across the axon membrane and the other to pass current across the membrane. The electrodes were attached to a feedback circuit, which applied whatever current was necessary to keep the voltage constant. With this device, we could monitor both the current and voltage, and thus determine the membrane conductance.
Allen: But Dr. Hodgkin, wasn’t the voltage clamp originally invented by Kenneth Cole at Woods Hole in the United States?
Hodgkin: Yes, he did some of the early work. And it’s true I visited Woods Hole and learned the technique from Cole. But some of his measurements were questionable. He reported action potentials that went positive by over 100 millivolts!
Einthoven: Oh my.
Hodgkin: An action potential that positive would have invalidated the idea that the membrane depolarized until it reached the sodium equilibrium voltage, which was the basis for the Hodgkin and Huxley model. In any case, we were able to calculate the membrane conductance and could determine how the membrane changed its conductance for both sodium and potassium individually.
Lauterbur: Because the sodium and potassium ions passed through selective ion channels?
Hodgkin: Yes, but we didn’t know it at the time. We just knew that two ions were involved: sodium and potassium. At rest the axon was primarily permeable to potassium and during the action potential it became permeable to sodium. We imagined that “gates” allowed the current to pass through the membrane: the m and h gates for sodium, and the n gate for potassium. It may sound a bit ad hoc, but our Hodgkin and Huxley model was really a gigantic curve-fitting exercise.
When the membrane was slightly depolarized—that is, when the membrane voltage was made slightly positive compared to its resting value—the m gate began to open, letting in sodium. The positive charge of the sodium ions raised the membrane voltage further, resulting in the m gate opening more, allowing more sodium to enter…
Einthoven: Positive feedback!
Hodgkin: Yes, the upstroke of the action potential is a positive feedback loop.
Lauterbur: But Alan, positive feedback can be explosive. What stopped the rise in membrane voltage?
Hodgkin: When the membrane voltage reached the sodium equilibrium voltage, there was no longer a tendency for sodium to enter the nerve. Even though there was more sodium outside the axon than inside, the high positive voltage in the axoplasm prevented any more positive sodium ions from diffusing in. That’s why Cole’s huge action potentials didn’t make any sense. Their reported action potentials went above the sodium equilibrium voltage.
Curie: But Dr. Hodgkin, what then caused the voltage to return to rest?
Hodgkin: Well, the n gate slowly opened, allowing potassium to leave the axon (carrying positive charge with it). But more importantly, the h gate slowly closed, preventing any further sodium current.
Einthoven: But if the h gate closes, would not that destroy the positive feedback loop of the action potential?
Hodgkin: Yes indeed. The closing of h made the axon “refractory.” It couldn’t fire another action potential until the h gate finally opened again and the membrane returned to its resting state.
Allen: Interesting, Dr. Hodgkin. And you made a mathematical model of this?
Hodgkin: Yes. Much of that was Andrew’s work.
Lauterbur: Don’t some people call Huxley the “greatest mathematical biologist ever”?
Hodgkin: They do. Our model required solving a set of nonlinear differential equations. This was back in the days before digital computers were available. You should’ve seen Andrew working that hand-held mechanical calculating machine to solve those equations numerically. Boy, did his fingers fly!
Allen: Dr. Einthoven, you also worked in bioelectricity. Perhaps you could tell us about your discoveries?
Einthoven: Well, I was the first to record the electrocardiogram, which is the electrical signal produced by the heart.
Lauterbur: I often had an ECG taken during my yearly physical.
Einthoven: Yes, it has become one of the most important diagnostic tools of modern medicine. But unlike Dr. Hodgkin, I didn’t have fancy voltmeters and oscilloscopes that I could use to measure electrical current. I had to invent an improved “string galvanometer.”
Hodgkin: Yes, yes. You passed a current through a wire in a magnetic field, causing a force on the wire proportional to the current.
Enithoven: Indeed, Dr. Hodgkin. We could not measure currents that changed too rapidly or that were too weak, but the device was sufficient to record the electrocardiogram. Unlike Hodgkin and Huxley, I could not insert an electrode into a heart cell, so I had to be content with measuring the voltage produced on the body surface by the electrical activity of the distant heart.
Curie: And wasn’t it you, Dr. Einthoven, who assigned the names P-wave, QRS-complex, and T-wave to the various electrocardiogram deflections?
Enithoven: Yes it was. The P-wave corresponded to the atria depolarizing, the QRS-complex to the ventricles depolarizing, and the T-wave to the ventricles repolarizing.
Allen: And what about the atria repolarizing?
Einthoven: That tiny signal was buried in the QRS-complex.
Lauterbur: And how did ya interpret your data?
Einthoven: I imagined that the heart produced a dipole, which just means current passed out of the heart cells at one point and reentered the cells at another, like an electric dipole made from two charges separated by a distance. Really, the ECG is produced by tiny dipoles associated with each cardiac cell, but there are billions of cells so I simplified the situation by representing the current source as a single dipole.
Hodgkin: A “toy model”!
Einthoven: Yes, sometimes we must make approximations to simplify a complicated situation so we can understand it better. A dipole is a vector, meaning it has a magnitude and a direction. To determine its direction, I placed electrodes on the left arm, right arm, and left leg. These three electrodes roughly form an equilateral triangle…
Lauterbur: Einthoven’s triangle!
Einthoven: Some people started calling it that, which was quite an honor. The signal from the three electrodes forming “Einthoven’s” triangle, if you will, determine the dipole direction.
Curie: Can’t the electrocardiogram be used to treat diseases?
Einthoven: Not really treat, but diagnose. The details of the electrical signal provide information about heart arrhythmias. Once you know the type of arrhythmia, then you can treat it properly.
Allen: I believe that our modern artificial pacemakers and defibrillators are what you are referring to.
Einthoven: Yes. These miraculous devices can use ECG recordings to determine the correct place and time to stimulate the heart to overcome the arrhythmia. It is all quite wonderful, but those devices were invented long after I had left the scene.
Curie: But they’re based on your work, Dr. Einthoven. We all owe you a great debt of gratitude.
Einthoven: And to you, Dr. Curie, for your work on…
Allen: Before we discuss Dr. Curie’s research, I would like to hear from Dr. Lauterbur about his studies that led to magnetic resonance imaging.
Lauterbur: Steve, I’d love to talk about it. Some nuclei have a property called “spin.” A nucleus with spin, such as that of the hydrogen atom, precesses, or rotates, about a magnetic field, with its precession frequency proportional to the magnetic field strength. This precession is the basis for nuclear magnetic resonance.
Now, the secret to magnetic resonance imaging is to apply a large, static magnetic field that causes the spins to precess, plus a magnetic field gradient that you can turn on and off. The gradient makes the magnetic field larger in one location than another; it maps magnetic field strength to position. This causes the precession frequency to also vary with position. It’s the frequency that we measure during MRI. Therefore, the gradient maps frequency to position, allowing you to determine a nucleus’s location from its frequency.
Hodgkin: Wonderful! Why didn’t they call the method “nuclear magnetic resonance imaging”?
Lauterbur: Ha! People are so afraid of the word “nuclear” that they dropped it and renamed the technique “magnetic resonance imaging,” or MRI. Some people have such irrational fears of anything having to do with the nucleus or radiation.
Curie: I know what you mean, I remember when…
Allen: Yes, but let Dr. Lauterbur finish his story.
Lauterbur: I remember the day I came up with the idea of using gradient fields to do MRI. I was sitting in a Big Boy restaurant and it just came to me: Eureka! I immediately scribbled the thought down on the only thing I had available: a paper napkin.
Hodgkin: I’m glad they didn’t use cloth napkins.
Lauterbur: Ha. Not likely at a Big Boy. I would’ve walked out with it if they had. So I built the first simple MRI machine and started creating images. I tried to publish my initial results in the journal Nature…
Einthoven: Ah, that English journal is one of the finest in all of science.
Lauterbur: Perhaps, but they initially rejected my manuscript.
Curie: Goodness!
Lauterbur: They thought my images were too fuzzy. But they were the very first magnetic resonance images, for crying out loud. I persisted and asked them to review it again. It was finally published in Nature, and the article became a classic. I believe you could write the entire history of science in the last 50 years in terms of papers rejected by Science or Nature.
Allen: Really?
Lauterbur: Yes. I tried to patent my ideas, but Stony Brook decided not to pursue it. Patents are expensive, and they didn’t expect the potential earnings justified the cost of the lawyers and filing fees.
Curie: What a mistake.
Allen: Can you tell us a little about your controversy with Raymond Damadian regarding the invention of MRI.
Lauterbur: Steve, I thought ya might bring that up [audience laughs awkwardly]. Yes, Damadian also was working on imaging using MRI. He was particularly interested in finding if signals from a tumor were different from normal tissue. It was nice work, but it didn’t contain the idea of using a magnetic field gradient to map frequency to position, which is the essence of my method. When Peter Mansfield and I each received the Nobel Prize for developing MRI, Damadian took out a full-page ad in the New York Times claiming the prize should have gone to him too! He was bitter about it all his life. But I think history is on my side.
Einthoven: What about Herman Carr?
Lauterbur: Carr’s work was a precursor to mine and he probably had a better claim to the Nobel Prize than Damadian did. I should’ve cited Carrr’s work. And Mansfield thought Erwin Hahn deserved a piece of the prize for his discovery of spin echo. Scientific discoveries and inventions are complex processes, and the credit must often be shared among many researchers.
Hodgkin: Yes, I know. Besides Huxley, my work was assisted by Bernard Katz and William Rushton among others.
Allen: Including Kenneth Cole.
Hodgkin: [Sighs] And Cole.
Allen: Now, Dr. Curie, could you tell us a little of your important work.
Curie: Yes. I’ve been waiting patiently for my turn [quiet laughter]. First a little background. I lived in an exciting time for science…
Einthoven: I believe all times are exciting for science.
Curie: I agree, but the end of the 19th century was a particularly exciting time for physics. In 1895 Wilhelm Rontgen discovered x-rays (a type of very high frequency electromagnetic radiation) and then in 1896 Henri Becquerel discovered radiation from uranium. I decided to examine uranium in more detail.
Lauterbur: Didn’t you find three types of radiation: alpha, beta, and gamma?
Curie: No, Dr. Lauterbur, that was found by my good friend Ernest Rutherford. But back to my work. I started with a ton of pitchblende (an ore containing uranium) and analyzed it chemically, separating the radioactive and nonradioactive parts. My husband Pierre was so interested in this work that he abandoned his own research to help me. After years of analysis, we finally ended up with less than a gram of a new element we named radium. It was highly radioactive. Along the way, we also discovered another element, polonium, which I mentioned previously.
Hodgkin: Those discoveries must’ve made you very famous.
Curie: Well, it did allow me to finally obtain my doctorate from the University of Paris. I was then invited to the Royal Institution in London to lecture about radioactivity. But since I was a woman, I was not allowed to speak, and Pierre alone gave the presentation [indignant murmur from the audience]. The committee that decides the Nobel Prize was going to award it to only Pierre and Henri Becquerel, but Pierre put a stop to that. I miss Pierre so much. In 1906, he was killed in a road accident. It was devastating…
Allen: I’m so sorry, Dr. Curie [Allen pats Curie on the shoulder].
Curie: But I soldiered on. Almost immediately x-rays and radium began to be used in medicine, creating the new discipline of medical physics. During the First World War, my daughter Irene and I developed x-ray imaging equipment mounted on trucks that could be used as mobile radiography units at the front. They were known as “petites Curies,” or “Little Curies.” [Curie smiles.]
Einthoven: Was not Irene herself a famous scientist?
Curie: Yes, Irene and her husband won their own Nobel Prize for discovering artificial radioisotopes.
Allen: I know this may be a delicate subject, but could you tell us about your relationship with physicist Paul Langevin?
Curie: Mr. Allen, I’m surprised you have so little tact [audience laughs nervously]. But I suppose if you must know, Paul was a former student of Pierre’s. He was married, but was estranged from his wife. After Pierre died, Paul and I were lovers. The press got a hold of the news (by that time I was quite famous) and started calling it the “Langevin affair.” They tortured me about that relationship.
Allen: Thank you, Dr. Curie, for sharing that difficult part of your past. [Looking up at the entire group] There is one thing all four of you have in common. You all won a Nobel Prize.
Lauterbur: Yes, you’ve already heard my embarrassing story about the prize. I received mine, jointly with Mansfield, for Physiology and Medicine.
Hodgkin: I also received mine in Physiology or Medicine, along with Huxley and neurophysiologist John Eccles.
Einthoven: Mine was for Physiology or Medicine too, but it was a solo award.
Curie: I’m so proud of you all. But gentlemen, do any of you have two Nobel Prizes [all laugh]?
Hodgkin: Yes, Dr. Curie, you were the first woman to receive a Nobel Prize, and you ended up with two: one in physics and one in chemistry [several young female members of the audience start stomping their feet and cheering for Curie; one yells “girl power!”].
Allen: [Laughing] With that thought, my friends, I fear we have run out of time. I hope you will join us again next week for another episode of Meeting of Minds.