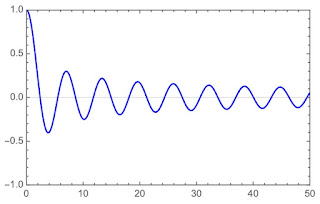
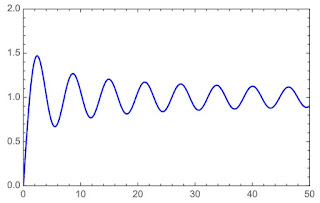
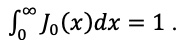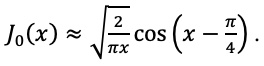|
| John Moulder, from Khurana et al. (2008) Med. Phys., 35:5203, with permission from Wiley. |
Moulder JE, Foster KR (1995) Biological Effects of Power-Frequency Fields as they Relate to Carcinogenesis. Proceedings of the Society for Experimental Biology and Medicine Volume 209, Pages 309–324.
Moulder JE, Foster KR (1999) Is There a Link Between Exposure to Power-Frequency Electric Fields and Cancer? IEEE Engineering in Medicine and Biology Magazine, Volume 18, Pages 109–116.
Moulder JE, Foster KR, Erdreich LS, McNamee JP (2005) Mobile Phones, Mobile Phone Base Stations and Cancer: A Review. International Journal of Radiation Biology, Volume 81, Pages 189–203.
Foster KR, Moulder JE (2013) Wi-Fi and Health: Review of Current Status and Research. Health Physics, Volume 105, Pages 561–575.Perhaps my favorite of Moulder’s publications is his Point/Counterpoint article in the journal Medical Physics.
Khurana VG, Moulder JE, Orton CG (2008) There is Currently Enough Evidence and Technology Available to Warrant Taking Immediate Steps to Reduce Exposure of Consumers to Cell-Phone-Related Electromagnetic Radiation. Medical Physics, Volume 35, Pages 5203–5206.Here is how Moulder is introduced in that paper.
Dr. Moulder obtained his Ph.D. in Biology in 1972 from Yale University. Since 1978, he has served on the faculty of the Medical College of Wisconsin, where he directs the NIH-funded Center for Medical Countermeasures Against Radiological Terrorism. His major research interests include the biological basis for carcinogenesis and cancer therapy, biological aspects of human exposure to non-ionizing radiation, and the prevention and treatment of radiation-induced normal tissue injuries. He has served on a number of national advisory groups concerned with environmental health, non-ionizing radiation, and radiological terrorism; and he currently serves as a radiation biology consultant to NASA.
 |
| Are Electromagnetic Fields Making Me Ill? |
Radiation biologist John Moulder, of the Medical College of Wisconsin, began maintaining a website titled “Power Lines and Cancer FAQs [frequently asked questions],” which exhaustively summarized the evidence pro and con. Although this website is no longer available online, an archived pdf of it is [13]. In a 1996 article published by IEEE Engineering in Medicine and Biology, Moulder reviewed dozens of studies, and concluded that:
Given the relative weakness of the epidemiology, combined with the extensive and unsupportive laboratory studies, and the biophysical implausibility of interactions at relevant field strengths, it is often difficult to see why there is still any scientific controversy over the issue of power-frequency fields and cancer. [14]
13. large.stanford.edu/publications/crime/references/moulder/moulder.pdf. Access date: January 12, 2022.
14. Moulder JE (1996) Biological Studies of Power-Frequency Fields and Carcinogenesis. IEEE Engineering in Medicine and Biology Magazine, Volume 15, Pages 31–49.
In a special issue of the International Journal of Radiation Biology dedicated to Moulder, Andrea DiCarlo and her colleagues discussed his work on radiological terrorism.
Through his awarded research grant and cooperative agreements from the NIH and beyond, John leaves behind a legacy of excellent, rigorous, and robust scientific findings, research collaborators who benefited from his expertise and dedication, and a cadre of well-trained students. Although it is impossible to list here all the lives that were touched, and the careers that were impacted by John’s influence, the authors can state with certainty that the field of medical preparedness for a radiation public health emergency would not be where it is now without the steadying hand and role played by Dr. Moulder, both in the early days in the program and during his final years as an active researcher. We are grateful for his years of research and join the entire radiation community in mourning the loss of a great investigator and person.John Moulder, you were a voice of reason in a crazy world. We’ll miss you.