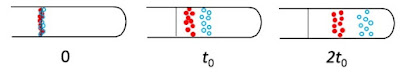
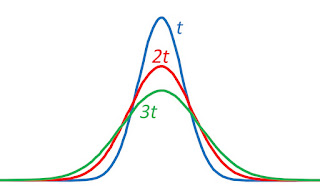
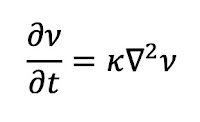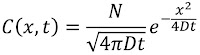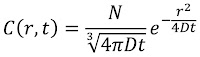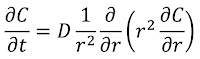The importance of turbulence (nonlaminar flow) is determined by a dimensionless number characteristic of the system called the Reynolds number NR. It is defined by
NR = L V ρ / η , (1.62)
where L is a length characteristic of the problem, V a velocity characteristic of the problem, ρ the density, and η the viscosity of the fluid.
 |
| Life in Moving Fluids, by Steven Vogel. |
The utility of the Reynolds number extends far beyond mere problems of drag; it’s the nearest thing we have to a completely general guide to what’s likely to happen when solid and fluid move with respect to each other. For a biologist, dealing with systems that span an enormous size range, the Reynolds number is the central scaling parameter that makes order of a diverse set of physical phenomena. It plays a role comparable to surface-to-volume ratio in physiology….I love the analogy to surface-to-volume ratio. Vogel continues
One of the marvelous gifts of nature is that this index proves to be so simple—a combination of four variables [L, V, ρ, and η], each with an exponent of unity. It has, however, a few features worth some comment. First, the Reynolds number is dimensionless… so its value is independent of the system of units in which the variables are expressed. Second, in it reappears the kinematic viscosity… What matters isn’t the dynamic viscosity, μ [Russ and I use the symbol η], and the density, ρ, so much as their ratio… Finally, a bit about L, commonly called the “characteristic length.” For a circular pipe, the diameter is used; choosing the diameter rather than the radius is entirely a matter of convention… The value of the Reynolds number is rarely worth worrying about to better than one or at most two significant figures. Still, that’s not trivial when biologically interesting flows span at least fourteen orders of magnitude[!]…Russ and I explain how the Reynolds number arises from the ratio of two forces, but I don't think we are as clear as Vogel.
Of greatest importance in the Reynolds number is the product of size and speed, telling us that the two work in concert, not counteractively. For living systems “small” almost always mean slow, and “large” almost always implies fast. That’s why the range of Reynolds numbers so far exceed the eight or so orders of magnitude over which the lengths of organisms vary…
What distinguishes regimes of flow is the relative importance of inertial and viscous forces. The former keeps things going; the latter makes them stop. High inertial forces favor turbulence… High viscous forces should prevent sustained turbulence and favor laminar flow by damping incipient eddies…The bacterium-whale comparison is just the sort of insight that Vogel excels at.
Another point should be made emphatically. If, for example, the Reynolds number is low, the situation is highly viscous. The flow will be dominated by viscous forces, vortices will be either nonexistent or nonsustained, and velocity gradients will be very gentle… If, in nature, small means slow and large means fast, then small creatures will live in a world dominated by viscous phenomena and large ones by inertial phenomena—this, even though the bacterium swims in the same water as the whale.
Tomorrow, I’ll provide a few more excerpts from Live in Moving Fluids, in which Vogel studies low Reynolds number flow in more detail.