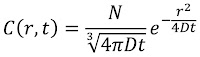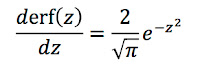Russ Hobbie and I analyze
Donnan equilibrium in Chapter 9 of
Intermediate Physics for Medicine and Biology.
Section 9.1 discusses Donnan equilibrium, in which the
presence of an impermeant ion on one side of a membrane,
along with other ions that can pass through, causes a potential
difference to build up across the membrane. This potential
difference exists even though the bulk solution on each side
of the membrane is electrically neutral.
Today I present two new homework problems based on one of Donnan’s original papers.
Donnan, F. G. (1924) “The Theory of Membrane Equilibria.” Chemical Reviews, Volume 1, Pages 73-90.
Here’s the first problem.
Section 9.1
Problem 2
½. Suppose you have two equal volumes of solution separated by a semipermeable membrane that can pass small ions like sodium and potassium but not large anions like A−. Initially, on the left is 1 mole of Na+ and 1 mole of A−, and on the right is 10 moles of K+ and 10 moles of A−. What is the equilibrium amount of Na+, K+, and A− on each side of the membrane?
Stop and solve the problem using the methods described in
IPMB. Then come back and compare your solution with mine (and Donnan’s).
In equilibrium,
x moles of sodium will cross the membrane from left to right. To preserve electroneutrality,
x moles of potassium will cross from right to left. So on the left you have 1 –
x moles of Na
+,
x moles of K
+, and 1 mole of A
−. On the right you have
x moles of Na
+, 10 –
x moles of K
+, and 10 moles of A
−.
Both sodium and potassium are distributed by the same
Boltzmann factor, implying that
[Na
+]
left/[Na
+]
right = [K
+]
left/[K
+]
right = exp(−
eV/
kT) (Eq. 9.4)
where
e is the
elementary charge,
V is the voltage across the membrane,
k is
Boltzmann’s constant, and
T is the
absolute temperature. Therefore
(1 –
x)/
x =
x/(10 –
x)
or
x = 10/11 = 0.91. The equilibrium amounts (in moles) are
left right
Na
+ 0.09 0.91
K
+ 0.91 9.09
A
− 1.00 10.00
The voltage across the membrane is
V =
kT/e ln([Na
+]
right/[Na
+]
left) = (26.7 mV) ln(10.1) = 62 mV .
Donnan writes
In other words, 9.1 per cent of the potassium ions originally
present [on the right] diffuse to [the left], while 90.9 per cent of the sodium
ions originally present [on the left] diffuse to [the right]. Thus the fall of
a relatively small percentage of the potassium ions down a
concentration gradient is sufficient in this case to pull a very
high percentage of the sodium ions up a concentration gradient.
The equilibrium state represents the simplest possible case of
two electrically interlocked and balanced diffusion-gradients.
Like this problem? Here’s another. Repeat the last problem, but instead of initially having 10 moles of K
+ on the right, assume you have 10 moles of Ca
++. Calcium is
divalent; how will that change the problem?
Problem 3
½. Suppose you have two solutions of equal volume separated by a semi-impermeable membrane that can pass small ions like sodium and calcium but not large anions like A− and B−−. Initially, on the left is 1 mole of Na+ and 1 mole of A−, and on the right is 10 moles of Ca++ and 10 moles of B−−. What is the equilibrium amount of Na+, Ca++, A− and B−− on each side of the membrane?
Again, stop, solve the problem, and then come back to compare solutions.
Suppose 2
x moles of Na
+ cross the membrane from left to right. To preserve electroneutrality,
x moles of Ca
++ move from right to left. Both cations are distributed by a Boltzmann factor (Eq. 9.4)
[Na
+]
left/[Na
+]
right = exp(−
eV/
kT)
[Ca
++]
left/[Ca
++]
right = exp(−2
eV/
kT) .
However,
exp(−2
eV/
kT) = [ exp(−
eV/
kT) ]
2
so
{ [Na
+]
left/[Na
+]
right }
2 = [Ca
++]
left/[Ca
++]
right
or
[ (1 –2
x)/(2
x) ]
2 =
x/(10 –
x)
This is a cubic equation that I can’t solve analytically. Some trial-and-error numerical work suggests
x = 0.414. The equilibrium amounts are therefore
left right
Na
+ 0.172 0.828
Ca
++ 0.414 9.586
A
− 1 0
B
−− 0 10
The voltage across the membrane is
V =
kT/e ln([Na
+]
right/[Na
+]
left) = (26.7 mV) ln(4.814) = 42 mV .
I think this is correct; Donnan didn’t give the answer in this case, so I’m flying solo.
 |
Frederick Donnan.
From an article in the Journal of Chemical Education,
Volume 4(7), page 819. |
Who was Donnan?
Frederick Donnan (1870 – 1956) was an Irish
physical chemist. He obtained his PhD at the
University of Leipzig under
Wilhelm Ostwald, and then worked for
Henry van’t Hoff. Most of his career was spent at the
University College London. He was elected a
fellow of the Royal Society and won the
Davy Medal in 1928 “for his contributions to physical chemistry and particularly for his theory of membrane equilibrium.”