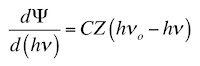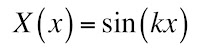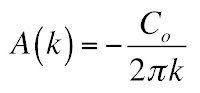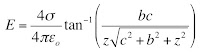In Chapter 18 of the 5th edition of
Intermediate Physics for Medicine and Biology,
Russ Hobbie and I discuss
magnetic resonance imaging. We describe how spins
precess in a magnetic field,
Bo =
Bo k, at their
Larmor frequency ω, and show that this behavior is particularly simple when expressed in a
rotating frame of reference. We then examine
radio-frequency excitation pulses by adding an oscillating magnetic field. Again, the analysis is simpler in the rotating frame. In our book, we apply the oscillating field in the laboratory frame’s
x-direction,
B1 =
B1 cos(ω
t)
i. The equations of the magnetization are complicated in the rotating frame (Eqs. 18.22-18.24), but become simpler when we average over time (Eq. 18.25). The time-averaged magnetization rotates about the rotating frame’s
x' axis with angular frequency γ
B1/2, where γ is the spin’s
gyromagnetic ratio. This motion is crucial for exciting the spins; it rotates them from their equilibrium position parallel to the static magnetic field
Bo into a plane perpendicular to
Bo.
When I teach medical physics (PHY 326 here at
Oakland University), I go over this derivation in class, but the students still need practice. I have them analyze some related examples as homework. For instance, the oscillating magnetic field can be in the
y direction,
B1 =
B1 cos(ω
t)
j, or can be shifted in time,
B1 =
B1 sin(ω
t)
i. Sometimes I even ask them to analyze what happens when the oscillating magnetic field is in the
z direction,
B1 =
B1 cos(ω
t)
k, parallel to the static field. This orientation is useless for exciting spins, but is useful as practice.
Yet another way to excite spins is using a
circularly polarized magnetic field,
B1 =
B1 cos(ω
t)
i –
B1 sin(ω
t)
j. The analysis of this case is similar to the one in
IPMB, with one twist: you don’t need to average over time! Below is a new homework problem illustrating this.
Problem 13 1/2. Assume you have a static magnetic field in the z direction and an oscillating, circularly polarized magnetic field in the x-y plane, B =Bo k + B1 cos(ωt) i – B1 sin(ωt) j.
a) Use Eq. 18.12 to derive the equations for the magnetization M in the laboratory frame of reference (ignore relaxation).
b) Use Eq. 18.18 to transform to the rotating coordinate system and derive equations for M'.
c) Interpret these results physically.
I get the same equations as derived in
IPMB (Eq. 18.25) except for a factor of one half; the angular frequency in the rotating frame is ω
1 = γ
B1. Not having to average over time makes the result easier to visualize. You don’t get a complex motion that—on average—rotates the magnetization. Instead, you get a plain old rotation. You can understand this behavior qualitatively without any math by realizing that in the rotating coordinate system the RF circularly polarized magnetic field is stationary, pointing in the
x’ direction. The spins simply precess around the seemingly static
B1'=
B1 i', just like the spins precess around the static
Bo =
Bo k in the laboratory frame.
Now let’s assume that an RF excitation pulse rotates the magnetization so it aligns with the
x' rotating axis. Once the pulse ends, what happens? Well, nothing happens unless we account for relaxation. Without relaxation the magnetization precesses around the static field, which means it just sits there stationary in the rotating frame. But we know that relaxation occurs. Consider the mechanism of dephasing, which underlies the
T2* relaxation time constant. Slight heterogeneities in
Bo mean that different spins precess at different Larmor frequencies, causing the spins to lose phase coherence, decreasing the net magnetization.
Next, consider the case of
spin lock. Imagine an RF pulse rotates the magnetization so it is parallel to the
x' rotating axis. Then, when the excitation pulse is over, immediately apply a circularly polarized RF pulse at the Larmor frequency, called
B2, which is aligned along the
x' rotating axis. In the rotating frame the magnetization is parallel to
B2, so nothing happens. Why bother? Consider those slight heterogeneities in
Bo that led to T
2* relaxation. They will cause the spins to dephase, picking up a component in the
y' direction. But a component along
y' will start to precess around
B2. Rather than dephasing,
B2 causes the spins to wobble around in the rotating frame, precessing about
x', with no net tendency to dephase. You just killed the mechanism leading to T
2*! Wow!
Will the spins just precess about
B2 forever? No, eventually other mechanisms will cause them to relax toward their equilibrium value. Their time constant will not be
T1 or
T2 or even T
2*, but something else called
T1ρ. Typcially, T
1ρ is much longer than T
2*. To measure T
1ρ, apply a 90 degree excitation pulse, then apply a RF spin lock oscillation and record the
free induction decay. Fit the decay to an exponential, and the time constant you obtain is T
1ρ. (I am not a MRI expert: I am not sure how you can measure a free induction decay when a spin lock field is present. I would think the spin lock field would swamp the FID.)
T
1ρ is sometimes measured to learn about the structure of
cartilage. It is analogous to T
1 relaxation in the laboratory frame, which explains its name. Because
B2 is typically much weaker than
Bo, T
1ρ is sensitive to a different range of
correlation times than T
1 or T
2 (see Fig. 18.12 in
IPMB).