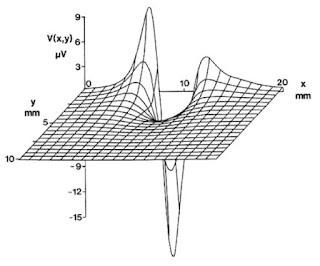
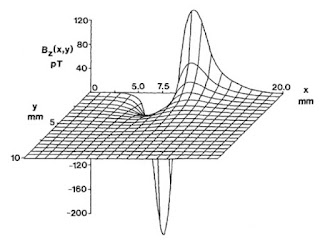
Buying fudge is a Mackinac Island tradition. We stopped at one of the iconic fudge shops, Murdick’s Fudge, and bought a few slabs. One of the Murdick clan, Ryan Murdick, attended Oakland University, where I teach, and obtained a master’s degree in physics. He and I published several papers together, including one about the bidomain model of the electrical properties of cardiac tissue (see Chapter 7 of the 4th edition of Intermediate Physics for Medicine and Biology), one about magnetocardiography (the magnetic field produced by the heart, Chapter 8), and one about how eddy currents induced in electroencephalogram electrodes can influence measurements of the magnetoencephalogram (Chapter 8; the effect on the MEG is very small). The papers are:
Murdick, R. and B. J. Roth (2003) “Magneto-encephalogram Artifacts Caused by Electro-encephalogram Electrodes,” Medical and Biological Engineering and Computing, Volume 41, Pages 203–205.I wasn’t expecting to find material for this blog on Mackinac Island, but I did. In 1822, Alexis St. Martin was accidentally shot in the abdomen in a small trading post near Fort Mackinac. Dr. William Beaumont was summoned to treat St. Martin, and was able to save his life. However, the wound healed in an odd way, leaving an opening providing access to the inside of his stomach.
Murdick, R. A. and B. J. Roth (2004) “A Comparative Model of Two Mechanisms From Which a Magnetic Field Arises in the Heart,” Journal of Applied Physics, Volume 95, Pages 5116–5122.
Roth, B. J., S. G. Patel, and R. A. Murdick (2006) “The Effect of the Cut Surface During Electrical Stimulation of a Cardiac Wedge Preparation,” IEEE Transactions on Biomedical Engineering, Volume 53, Pages 1187–1190.
Readers of the 4th edition of Intermediate Physics for Medicine and Biology will appreciate what happened next. The resourceful Beaumont took advantage of the situation to conduct experiments on digestion. He tied different foods to a string, threaded them into St. Martin’s stomach, left them to digest for a while, and then pulled them out to see what had happened. These ground-breaking experiments were instrumental in establishing how digestion works. I toured a small museum dedicated to Beaumont, which describes these experiments in graphic (perhaps too graphic) detail.
I am interested in Beaumont not just because of his experiments studying digestion. Beaumont Hospital, in Royal Oak Michigan, is the clinical partner for a new medical school recently established at Oakland University. The first class of students at the Oakland University William Beaumont School of Medicine arrives this August. This will be a landmark event in OU’s history, and we are all excited about it.
I can’t help but be intrigued by the juxtaposition of these two stories: William Beaumont’s experiments on Alexis St. Martin, and the establishment of a new medical school bearing Beaumont’s name. St. Martin lived into his 80s. I expect our new medical school will have a similarly long and productive life.